Massachusetts based Lilliputian Systems Inc. has developed a portable butane powered fuel cell meant to serve as a standalone recharger for devices such as smart phones and laptops. The fuel cell itself is about the size of an iPhone and uses replaceable butane cartridges good for 16-20 recharges for a smart phone such as the iPhone or BlackBerry. The cost is said to be around $200 for the fuel cell and $1 to $4 for the butane cartridges.
Other portable fuel cell devices developed by other companies promised similar offerings but have yet to bring a viable product to market. The main challenges other companies faced were the fuel type used and the heat generated by the reaction. Lilliputian is using butane which contains roughly 70% more energy per gram than methanol which is commonly used in proton exchange membrane (PEM) type fuel cells.
PEM fuel cells operate at around 80°C with a relatively quick startup time and cause less wear on the system. The issue with PEM fuel cells is that to date they have all required the precious metal platinum for the catalyst to strip the hydrogen of its electrons. Platinum adds to the cost of the system and is also susceptible to carbon monoxide “poisoning” which then requires an additional reactor to help reduce carbon monoxide from the system.
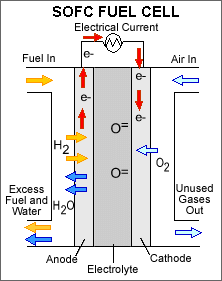 Solid oxide fuel cells are the type that Lilliputian is using in their devices. Solid oxide differs from PEM primarily in the type of electrode it uses. A hard, non-porous, ceramic compound is what is used for the electrode in a solid oxide fuel cell. This allows for the use of more energy dense fuels such as butane and even carbon monoxide which does not negatively affect the solid oxide electrode like the platinum one in a PEM fuel cell. The big drawback to solid oxide is the operating temperature required. Temperatures as high as 1000°C are generated and require thermal shielding to prevent injuries. These high temperatures are also taxing on the components in the system causing significantly more wear than the mild 80°C temperature produced by a PEM fuel cell.
Solid oxide fuel cells are the type that Lilliputian is using in their devices. Solid oxide differs from PEM primarily in the type of electrode it uses. A hard, non-porous, ceramic compound is what is used for the electrode in a solid oxide fuel cell. This allows for the use of more energy dense fuels such as butane and even carbon monoxide which does not negatively affect the solid oxide electrode like the platinum one in a PEM fuel cell. The big drawback to solid oxide is the operating temperature required. Temperatures as high as 1000°C are generated and require thermal shielding to prevent injuries. These high temperatures are also taxing on the components in the system causing significantly more wear than the mild 80°C temperature produced by a PEM fuel cell.
Lilliputian addresses the heat issue by placing the solid oxide electrode on a layer of silicon then surrounding it with a bubble to create a vacuum in. This vacuum works much like an incandescent light bulb that has a filament heated to around 2500°C. The device on the outside of its thermal shielding and packaging should feel about the same as room temperature.
The main question now is even if their device does everything they say it can, will people want to use this type of product as opposed to their standard Li-ion battery. I personally don’t envision myself wanting to have a device completely dependent on this type of energy source but can foresee military applications that would. To create a market they will need to find a niche first and grow from there while continuing to improve upon their products’ performance and safety to help it become more broadly accepted by the masses.

